1. Post a picture of three 3-dimensional Ball and Stick molecular models(choose your three favorite molecules) that you have created with common items around your home. Also post a molecular structure image(image from the web, of either a Kekule Structure or a Ball and Stick Model) and the IUPAC name of the molecule.
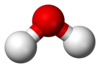
Water
IUPAC: Oxidane
Methane
IUPAC: Tetrahydridocarbon
IUPAC: methanedione
2. Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat. Explore the ingredients of things like cosmetics and foods.
- Water
Oxidane
H20
- Salt

Sodium Chloride
NaCl
- Ammonia

Azane
NH3
- Bleach

Sodium Hypochlorite
NaClO
- Baking Soda
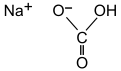
Sodium hydrogen carbonate
| NaHCO 3 | |
- Vinegar

Acetic acid
C2H4O2
- Alcohol

Ethanol
C2H5OH
- Rubbing alcohol

Isopropyl alcohol
C3H8O
- Propane
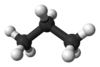
Propane
C3H8
- Sugar
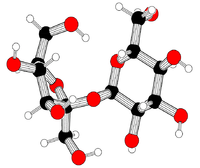
(2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
C12H22O11
- Fertilizer
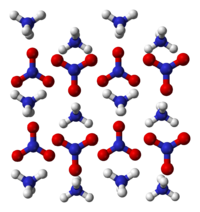
Ammonium nitrate
(NH4)(NO3)
- Citric Acid

2-hydroxypropane-1,2,3-tricarboxylic acid
C6H8O7
- Hydrogen Peroxide

dihydrogen dioxide
2(HO)
- Tums

Calcium Carbonate
CaCO3
- Aspirin

2-acetoxybenzoic acid
| C9H8O4 |
- Acetone

Propanone
C3H6O
- Carbon Dioxide

Carbon Dioxide
CO2
- Borax

Sodium tetraborate decahydrate
Na2B4O7·10H2O or Na2[B4O5(OH)4]·8H2O
- Tylenol

N-(4-hydroxyphenyl)ethanamide
N-(4-hydroxyphenyl)acetamide
C8H9NO2
- Silica
Silicon dioxide
SiO2
3. Look over your molecules and the bonding characteristics, how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon: 4 bonds
Hydrogen: 1 bond
Oxygen: 2 bonds
4. What does IUPAC stand for?
International Union of Pure and Applied Chemistry
5. As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be? Here is an example:
http://www.naturalhealthcareproducts.com/Cleaning-Products.php
I don't really think that any product, especially a cleaning product can be chemical free. At the very least, there has to be water in the product. I think that people are trying very hard to be clean and 'green' and that makes companies feel that it is necessary to exploit the idea that their product either has less chemicals or maybe less potentially harmful chemicals. However, I find that it is a far stretch of the truth to call anything, especially a cleaning product, chemical free.
Do a little web searching and propose what chemicals are actually in this product. Keep in mind, that everything at the molecular level is a chemical, whether it be made in nature or in a lab.




No comments:
Post a Comment